It is important to Limeway that our products are science-based, this including a solid Therapeutic Concept. The diagram below shows our earliest attempt to capture these elements.
Dermatological Design Map: Integrated Design
2% Tranexamic cream for the treatment of Melasma (licensed from Limeway in 5 Asian Markets).
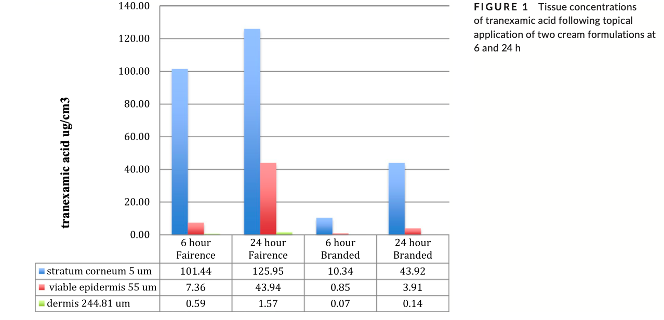
Attached is a .pdf of J Cosmet Dermatol. 2020;00:1–7. In vitro human skin concentrations following topical application of 2% tranexamic acid in co-enhancer cream and branded cream formulations. Briefly, tranexamic acid inhibits melanin synthesis via inhibition of the plasminogen-plasmin system, so has clinically relevant biology. Although via oral administration tranexamic acid is clearly clinically effective, the evidence is weak following topical application. However, pharmacokinetic modelling estimates of free drug concentration in the basal epidermis predict the potential for efficacy via the topical route. Figure 1 shows tranexamic skin concentrations following topical application of two 2% tranexamic creams in vitro to human skin. Epidermal concentration from Fairence were significantly greater than from the comparator cream and within the concentration range predicted for efficacy. A clinical study conducted in melasma patients confirmed this expectation.

5% Lactobionic acid for low pH-mediated inhibition of serine protease and itch.
Puritus, itch, is a major unmet need. Polyhydroxy acids, such as lactobionic acid (LBA), relatively new cosmetic technologies, are considered to have superior skin tolerance to traditional lactic/glycolic acids and similar efficacy. It was reasoned that the Limeway co-enhancer technology could be used to increase efficacy of LBA whilst retaining skin tolerance. Supporting the hypothesis, Hachem J-P et al. (JID 2010; 130(2): 500-10) reported that topical application of LBA very significantly reduced pH in mouse stratum corneum to restore the skin barrier.
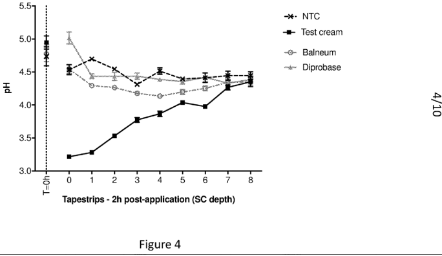
Figure 4, from AU2018267267 shows the ability of 5% lactobionic acid in a Limeway co-enhancer cream base to reduce pH in-vivo within human stratum corneum to inhibit protease activity.
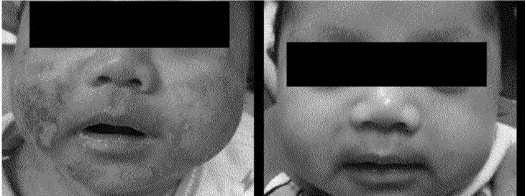
This product is currently patented / marketed in Asia, with other patents filed internationally by our client including China, Australia, New Zealand and the USA.